centurion
Products
Setting out with the motto that "every patient deserves the chance of receiving modern treatment and high quality of life", our company provides the best treatment options in its therapeutic area for patients of all age groups, improving their quality of life and contributing value to healthcare.
MORE

centurion
Manufacturing
As Centurion Pharma, we are of the few pharmaceutical companies in Turkey with unique portfolio of sterile injectables placed in Ankara Başkent Organized Industrial Zone. Our manufacturing systems are fully equipped with state of the art technology and with our expertise and know how we are in compliance with international standards.Our facility has s received EU GMP certificate in 2021 from Bulgarian Drug Agency.
MOREcenturion
R&D
For our R&D Center, which sets out with the motto of “Every patient deserves the chance of receiving a modern treatment and the high quality of life”, humans and quality are the most important milestones.
MORE
centurion
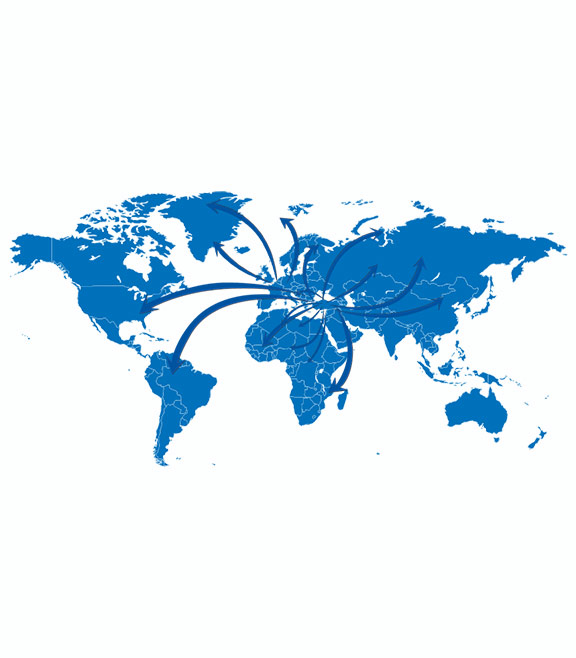
Our Business Partners
As Centurion Pharma, we have been cooperating with many prominent organizations and pharmal companies since 1979 targeting treatment for unmet medical needs of patients in Turkey and many parts of the World.
MORE






